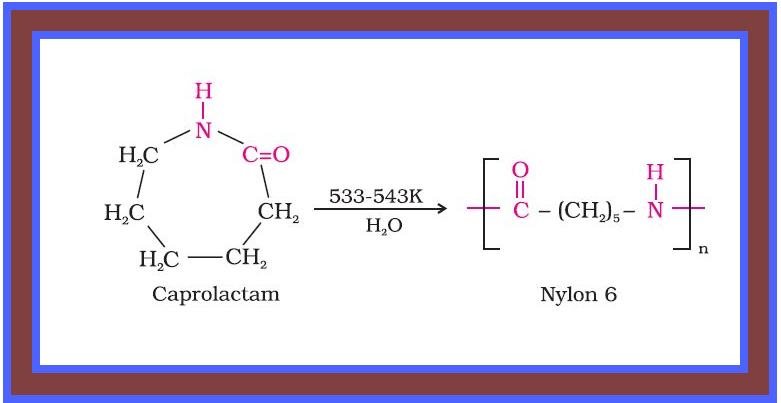
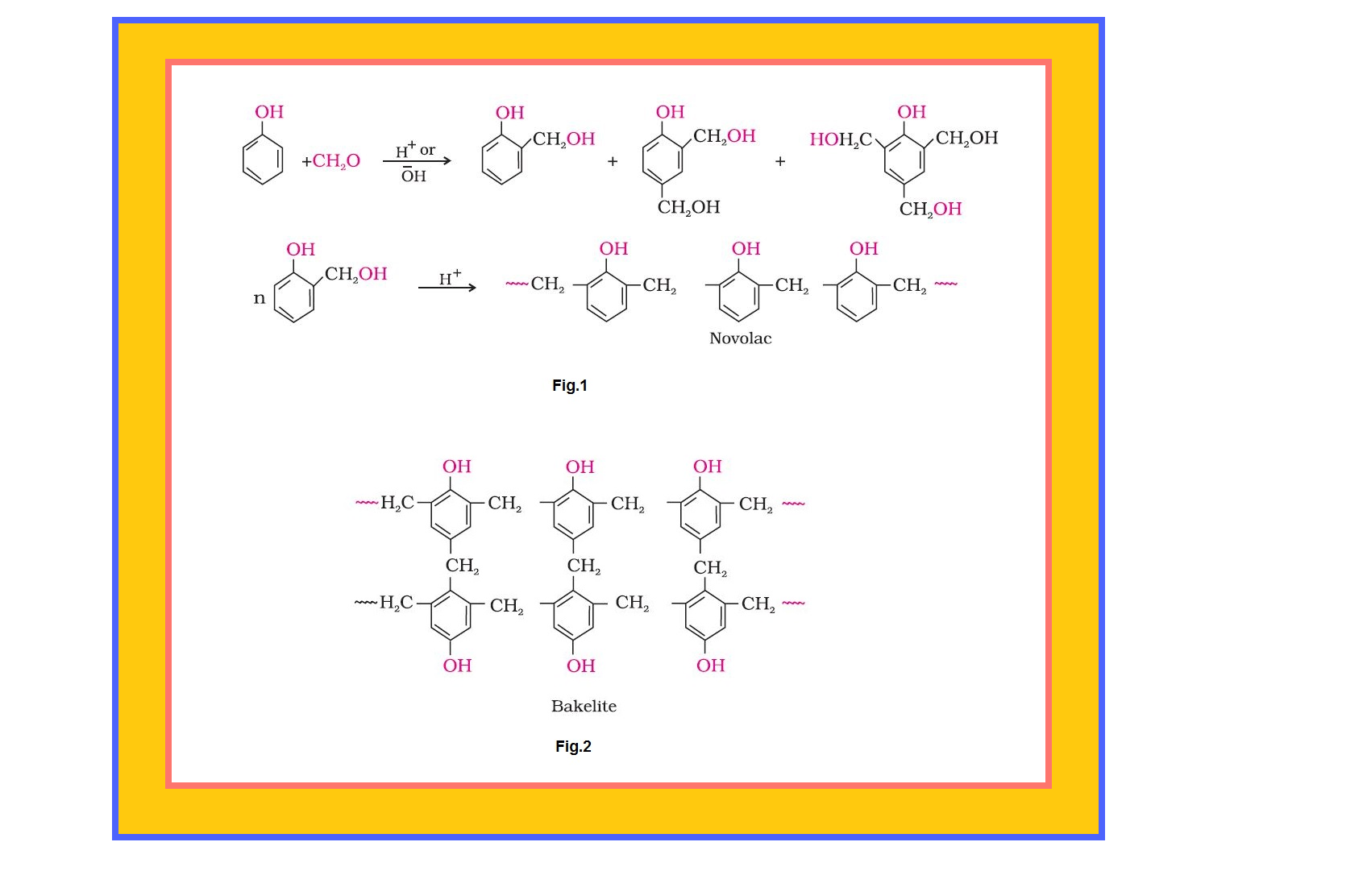
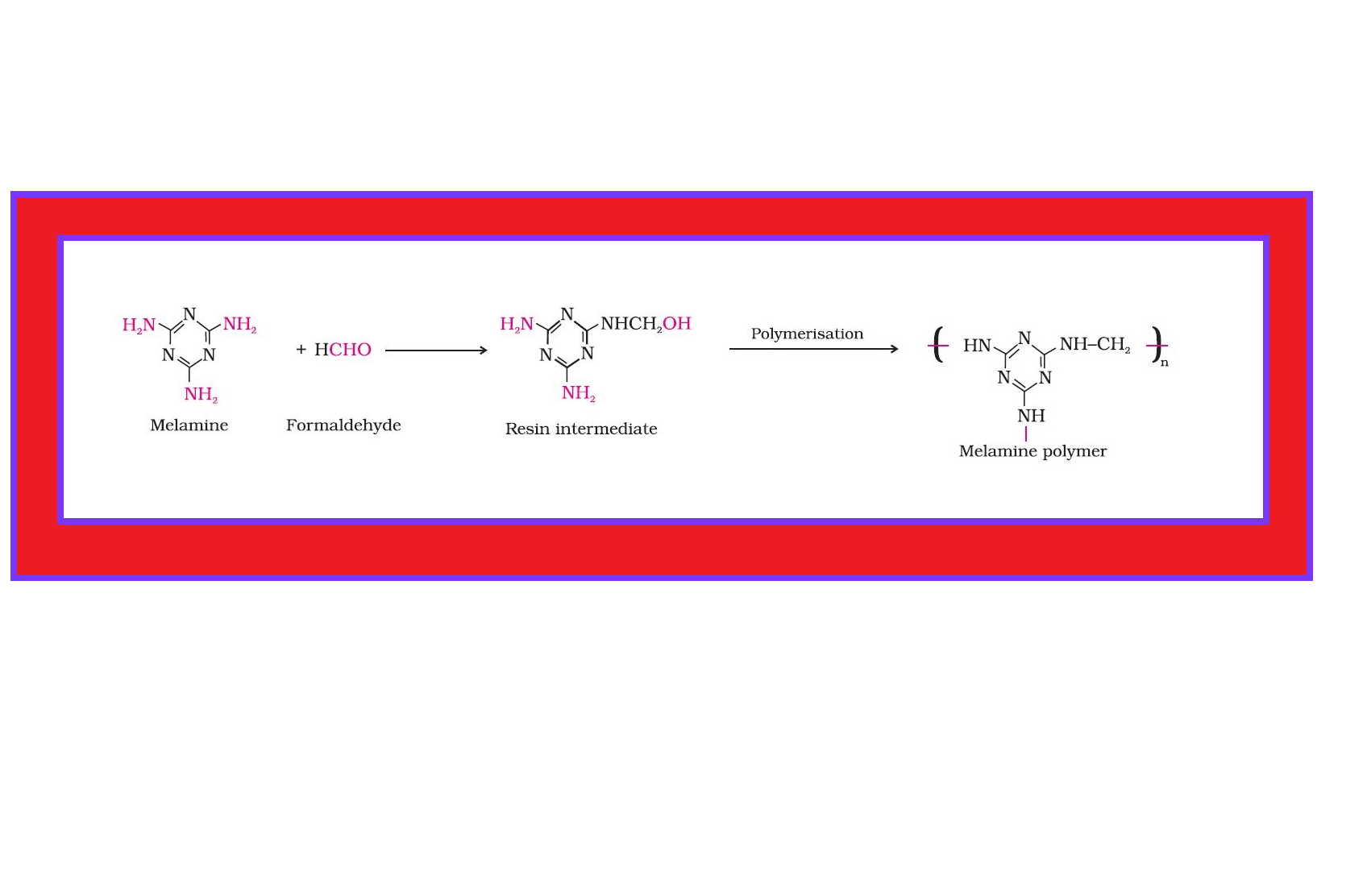
Condensation Polymerisation or Step Growth polymerisation :
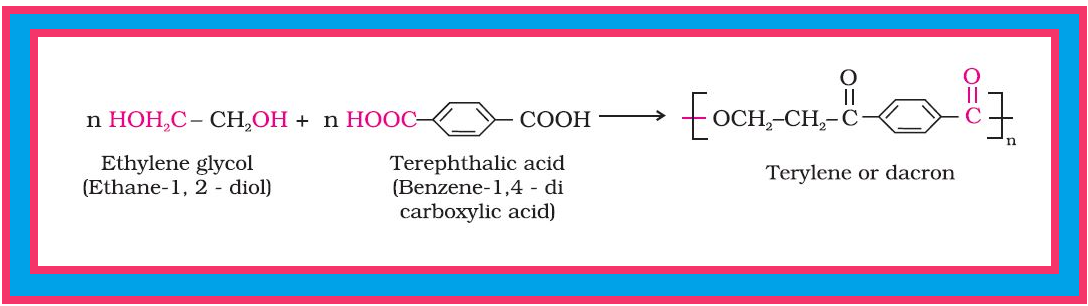
`=>` This type of polymerisation generally involves a repetitive condensation reaction between two bi-functional monomers.
`=>` These polycondensation reactions may result in the loss of some simple molecules as water, alcohol, etc., and lead to the formation of high molecular mass condensation polymers.
`=>` In these reactions, the product of each step is again a bi-functional species and the sequence of condensation goes on.
`=>` Each step produces a distinct functionalised species and is independent of each other, this process is also called as step growth polymerisation.
`color{red}("Example ")` : The formation of terylene or dacron by the interaction of ethylene glycol and terephthalic acid.
Some important condensation polymerisation reactions characterised by their linking units are described below :
`=>` These polycondensation reactions may result in the loss of some simple molecules as water, alcohol, etc., and lead to the formation of high molecular mass condensation polymers.
`=>` In these reactions, the product of each step is again a bi-functional species and the sequence of condensation goes on.
`=>` Each step produces a distinct functionalised species and is independent of each other, this process is also called as step growth polymerisation.
`color{red}("Example ")` : The formation of terylene or dacron by the interaction of ethylene glycol and terephthalic acid.
Some important condensation polymerisation reactions characterised by their linking units are described below :